.
Soap is the oldest and most used washing detergent in the history of mankind. The oldest soap recipe that has been found is thousands of years old! And the best thing is: we still know how to make soap. The process differs a bit from that of our ancestors; after all, who would want to make lye from wood ash and boil it for hours with animal fats until it becomes a (stinky) bar of soap? The chemical principle or rather, the science behind soapmaking, however, stays the same and understanding the science behind it helps you to avoid mistakes, create your own recipes and stay safe during the process.
I was never good at chemistry, however. On the contrary, all things chemistry have always been a bit like magic for me. I mean, you take one thing and bring it together with another and you get something completely different! Weird, huh?
In this post, therefore, I will explain all this science stuff very simply – because that’s how I get it best. And I hope you, too.
The science behind soapmaking: What is soap?
Let’s start with the most mundane question: What is soap?
Soaps are surfactants and are used in combination with water as a cleaning agent. Surfactants are wash-active substances that reduce the surface tension of liquids. By that, they cause two normally immiscible substances, for example fat and water to unite. Why is that so? A fat molecule consists mostly of long chains of carbon and hydrogen atoms. The electricity is evenly distributed in these chains and therefore fat molecules are electrically non-polar.
Water, on the other side, consists of a negatively charged oxygen atom and two positively charged hydrogen atoms. Due to their structure, one side of the water molecule is negatively and the other is positively charged. This uneven distribution of charge is called “polar”. The negatively charged oxygen side attracts the positively charged hydrogen side of another water molecule like a magnet which is why water molecules are usually formed into clusters. They can’t, however, connect with the non-polar fat molecules.
Soap, now, works as an “agent” between fat and water on their molecular basis. The soap molecules have a water-attracting (hydrophilic) and a water-repellent (hydrophobic) side.
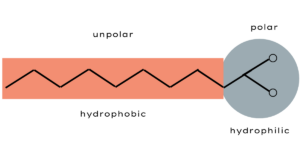
The hydrophobic side attracts fat and encloses the fat droplets in small circles. The hydrophilic end connects to water. The result is an oil-in-water mixture, a so-called dispersion.
By washing (that is rubbing your hands), the fats contained in the dirt come off and the fat-dirt-dispersion is rinsed off with water.
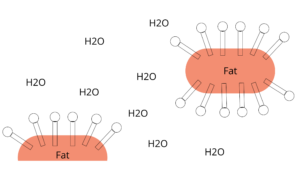
I told you: it’s magic!
What is soapmaking?
I’d love to have a time machine and go back to that one day when someone discovered by accident that by cooking fats with alkaline pot ash you’d get a washing-active substance that dissolves dirt and fat.
The principle of breaking down fats with alkaline substances (that is: lyes) has not changed since that first day of soapmaking. Today, we know the chemical reaction of soapmaking, in chem-talk also known as saponification. As lyes, we use sodium hydroxide or potassium hydroxide. When saponification is carried out with sodium hydroxide we get hard soaps, with potassium hydroxide we get soft to semi-soft soaps. The lye breaks down the fat into soap (or water-soluble alkaline salts) and glycerin, or, in chem-talk:
Fat + Lye = alkaline salt (soap) + glycerin
Plainly said: Fats react with lye to create a soap with a small amount of glycerin through saponification.
In soapmaking for skin and hair, we use a sodium hydroxide solution as a lye. Sodium hydroxide can be bought as crystals or flakes and it needs to be dissolved in a liquid (mostly water) so that we can mix it with the oil.
Saponification value
When lye is added to a liquid, an exothermic (=heat-producing) reaction occurs. It’s fascinating to watch the red bar on the thermometer climbing up! When we use water, temperatures reach about 90 – 100 °C, when other liquids are used the temperature is often hotter.
The amount of lye needed to make soap depends on the oils used and is called the saponification (SAP) value. Oils are made of short- and long-chain fatty acids which dictate the amount of lye. For example, coconut oil contains about 85 percent saturated fatty acid which is responsible for the fact that palm oil is solid at room temperature. Olive oil, on the other hand, contains mostly unsaturated fatty acid which makes it liquid at room temperature. Coconut oil reacts radically differently than olive oil when mixed with sodium hydroxide and thus, both oils require different amounts of lye to turn them into soap.
Superfatting
As described above, soap works by clinging its lather to dirt, both of which are rinsed away by water. This process, however, can also take away the skin’s natural oils. To prevent the skin from drying out after washing, we add extra oil to the soap, a process called “superfatting” or “lye discounting”.
If we make soap with a recipe that uses the exact amount of lye that is necessary to turn all the oil into soap has a zero percent lye discount or is zero percent superfat. That means it has no excess oil after saponification which makes it a hard bar of soap but is not very gentle to the skin.
A soap with excess oil, on the other hand, makes a softer bar of soap that has a decreased shelf life.
Most soapmakers keep their superfat to under 10 percent, I mostly use superfats between three (hair soap) and nine percent.
Different methods of soapmaking
There are different methods of soapmaking.
Hot-Process
To accelerate the breaking down of the fats, we heat the soap batter to 100 °C, accelerating the reaction as well as water vaporization. When the soap has cooled down, the process of saponification is finished.
I haven’t tried out this process yet but it’s only a question of time.
Cold-Process
Here, no external heat is added but the chemical reaction releases heat (= exothermic reaction). Saponification takes a longer time (24 – 48 hours) and we have to leave the soap for that time in a mould. Afterwards, the soap must be cured for several weeks before it is ready to use.
This is the classic method for making body and face soaps and I have loved it since the first soap I made!
Melt and pour
With that method, you melt a premade soap base, customize it with fragrances and colourants and pour it into a mould.
This is a method I don’t use.
The science behind soapmaking: Ingredients
As described above, soap consists of fats and lye and – if you like – additives. Let’s have a closer look:
Fats
Our basic raw materials are oils or fats of plant or animal origin. The most commonly used fats/oils for soapmaking have a high content of saturated and monounsaturated fatty acids like olive oil, coconut oil, palm fat, babassu oil and high-oleic oils like sunflower oil, rapeseed, peanut and safflower oil.
These fatty acids make solid soaps with a good storage time.
By a skilful combination of different fats and oils, the soaps’ characteristics can be optimized. That’s why we use mostly mixtures of fat/oils.
Lye
To produce solid hand and body soaps we saponify fats/oils a lye made of water and sodium hydroxide (NaOH). This lye has a pH value of 14 and therefore is strongly alkaline. NaOH is a white hygroscopic (i.e. water-attracting) solid and can be bought as crystals or flakes.
Any lye is a hazardous substance! You must know the risks and dangers and be very careful when working with it!
Additives
Depending on the purpose of our soap, we can add additives to optimize its properties or enhance its sensory effects. However, additives should be used sparsely and only in the best possible quality.
Fragrances
Although fragrances make only a small part of the overall soap amount, they determine (together with colourants) the first impression and influence our perception.
Essential oils

Essential oils are mostly gained by water vapour distillation of aromatic plants. Their shelf life varies with oils from citrus fruit having the shortest. We differentiate:
- untreated essential oils (gained from the plant)
- natural essential oils (consisting of several untreated components which haven’t all been gained from the plant it’s named after; no synthetic additives)
- nature identical essential oils (synthetic oils, composed acc. to a natural model)
- natural / nature-identical essential oils (mixtures of untreated and synthetic oils)
- artificial essential oils (artificial fragrances; often classified as health critical
Essential oils are irritating to skin and mucous membranes (work safety!) and may cause allergies.
Synthetic fragrances
Nowadays, synthetic fragrances are commonplace. Production is cheap, they have a long shelf-life and replace essential oils in many products. However, synthetic fragrances are chemicals and may contain components that are harmful to the organism. they may lead to contact allergies and allergies through inhalation. They degrade slowly and accumulate in the air.
Fragrancing of soaps
Fragrances, even in a low percentage, influence the physical properties of soaps, for example their solubility, emulsification, hardness and brittleness. It is therefore recommended, to use 2 – 3 percent (related to the whole fat amount) at the most. In soaps or sensitive skin or children, fragrances should not be used at all.
All fragrances used in soapmaking must be alkali-resistant, that is they must not be affected by the lye. Additionally, they must withstand the temperature at soapmaking. Fragrances based on alcohol are unsuitable as alcohol disrupts saponification.
Colour additives

Chemically speaking, we differentiate between inorganic and organic colour additives. Further differentiation is made according to the colourant’s capability to dissolve in the application medium (soap, water or oil).
Colour additives are classified as chemicals and may cause contact allergies. Some are even toxic or are classified as environmentally dangerous. When using colour additives, you must observe the safety instructions.
Colourants
Colourants are chemical compounds that dissolve in the application medium and dye other materials. There are natural and synthetic colourants. Natural colourants can be of animal or plant origin, with animal colourants deriving from bodily fluids like gall or blood and plant colourants deriving from woods, barks, roots, fruits, leaves and seeds. Their quality depends on climate, area, soil properties, etc.
Synthetic colorants on the other hand show a consistent quality as they are made under standardized production conditions. They are light- and heat-resistant and have an almost unlimited shelf-life.
Pigments
Pigments are colouring substances in the form of very fine particles that don’t dissolve in the application medium. The smaller the particles the more intense the colouring. Pigments can either be inorganic or organic, natural or synthetic.
- Anorganic natural pigments are obtained from soils and minerals.
- Inorganic synthetic pigments are produced with different chemical procedures and in high quantities, for example iron oxide pigments, metal effect pigments, white pigments (e.g. titanium oxide)
- Organic natural pigments are made of animal and plant parts
- Organic synthetic pigments are unsoluble
Water
For soapmaking, normal, still water is good. Tap water is ok as long as it doesn’t contain too much lime. Alternatively, you can use hydrolates, and alcoholic beverages like wine beer or milk (of animal or plant origin). With alcohol and milk, however, the soapmaking process is slightly different as these two heat too much and thus must be treated differently.
Resume
You see now, as simple as soapmaking may be at its core, it’s also very flexible and adaptable. By mixing different oils, using alternative liquid possibilities, and adding fragrances and colours you have almost infinite possibilities to create your own recipes.
I’m sure you can’t wait to learn how to make your first soap, right?! Get acquainted with the safety instructions, look how it works and then: make your first soap with this easy beginner-friendly recipe!


0 Comments